Pharmacovigilance
Monitoring Medicines, Maximizing Safety-Our Pharmacovigilance Promise
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem.
The aims of PV are to enhance patient care and patient safety in relation to the use of medicines and to support public health programmes by providing reliable, balanced information for the effective assessment of the risk-benefit profile of medicines. (Reference: WHO)
Pharmacovigilance System Of Radiant Was Set Up In 2013
Department of Pharmacovigilance of Radiant Pharmaceuticals Limited performs all Pharmacovigilance related activities of Radiant Pharmaceuticals Limited, Radiant Nutraceuticals Limited, Radiant Business Consortium Limited, Radiant Export Import Enterprise, Radiant Distributions Limited and Pharmacil Limited
A group of doctors and pharmacists are presently working at this department.
Department of Pharmacovigilance maintains separate PV QMS (Pharmacovigilance Quality Management System) to carry out all PV functions including processing ICSRs, monitoring local literature, providing PV awareness training to all employees, communicating & reporting to local Regulatory Authority as well as our Business Partners (F. Hoffmann-La Roche, Boehringer Ingelheim, Cheplapharm, Ferring, Fresenius Kabi, Pfizer, Wockhardt).
Pharmacovigilance
Required Information For Creating An AE Report
An adverse event report should contain the four essential information
All Serious & Non-serious Adverse Event (AE) or Adverse Drug Reaction (ADR) related to the use of Radiant’s Products (manufactured, imported and/or distributed) should be reported to the Department of Pharmacovigilance.

Patient’s Details
Name / Initials
Gender, age
Contact number
Pregnancy status
Reporter’s Details
Name
Occupation
Address
Contact number
Adverse Event
Date of onset
Symptoms
Outcome
Seriousness
Medical history
Suspected Product(s)
Brand name
Dose, frequency
Route
Batch number
Other medications
An Overview of The Adverse Event Report Form
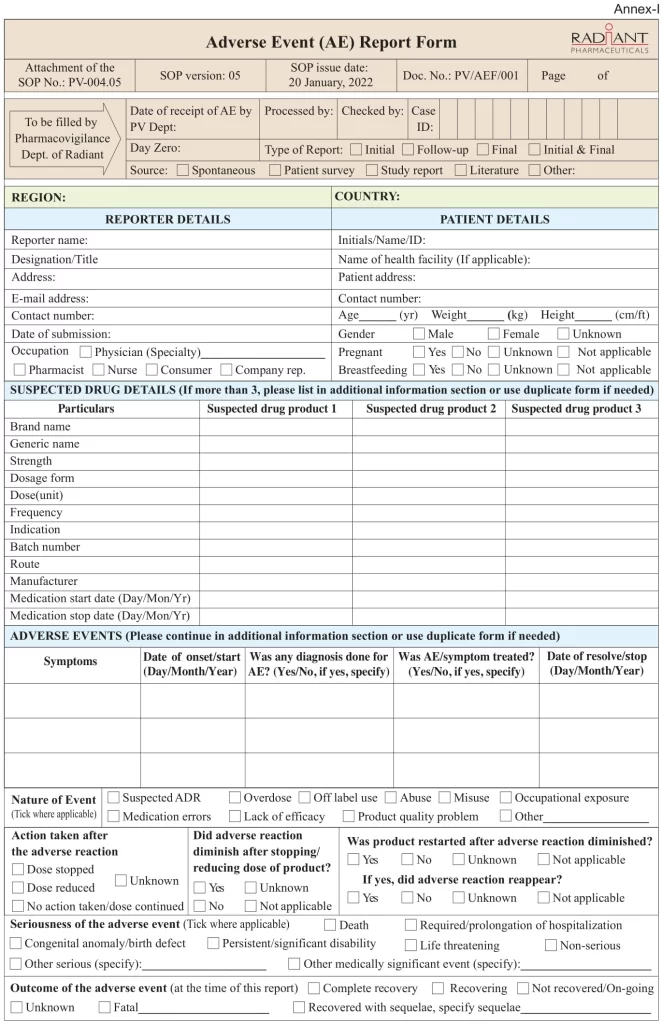
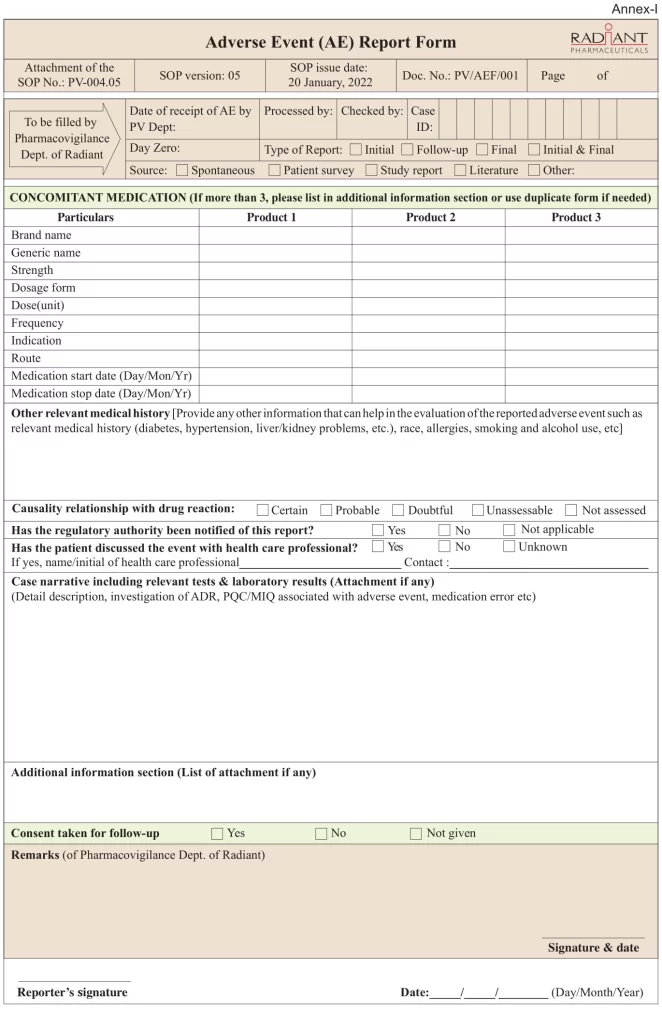
All consumers or patients are advised to contact their doctor for advice on medicines or any health problems/ medical emergencies. Pharmacovigilance enhance safer use of medicine by monitoring the safety and efficacy of medicines and by providing reliable, balanced information for the effective assessment of the risk-benefit profile of medicines.
Confidentiality Note: Any information related to the identities of the patient and reporter will be kept confidential unless it is subject to disclose due to regulatory obligation.
How To Report
Any adverse event regarding Radiant’s Products (manufactured, imported and/or distributed) can be reported to the Department of Pharmacovigilance.
Newsroom
Discover our contributions, perspectives and latest products
We are constantly bringing innovative, life-saving solutions to the industry and spreading medical awareness to professionals and civilians alike
Radiant Affiliate Companies
Strength in collaboration,
synergy in action
Explore and discover Radiant's collaborative ecosystem
Radiant Pharmaceuticals Limited
Radiant Nutraceuticals Limited
Jenphar Bangladesh Limited

Radiant Export Import Enterprise

Radiant Business Consortium Limited

Radiant Distributions Limited
Pharmacil Limited

Radiant Oncos Molbiol Limited

Shamutshuk Printers Limited

AeroMate Services Limited

AeroWing Aviation Limited

Radiant Care Limited


















